Disease Overview1,2
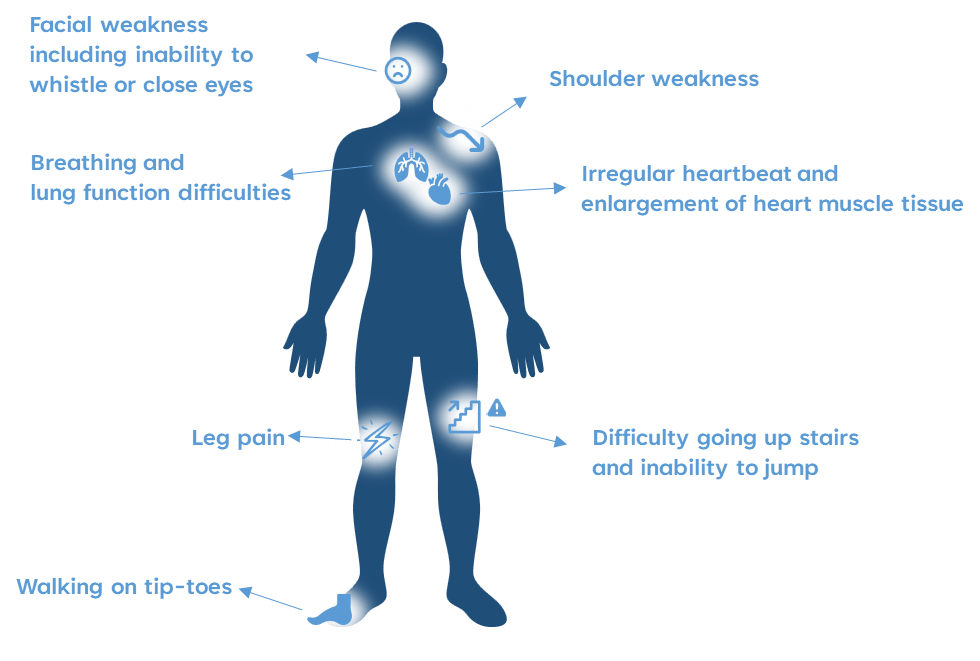
Duchenne muscular dystrophy (DMD) is an X-linked recessive neuromuscular disorder that causes progressive muscle weakness and wasting. It affects approximately 1 in 3600 to 9300 male newborns.
Common symptoms of DMD include:
The unmet need in DMD2
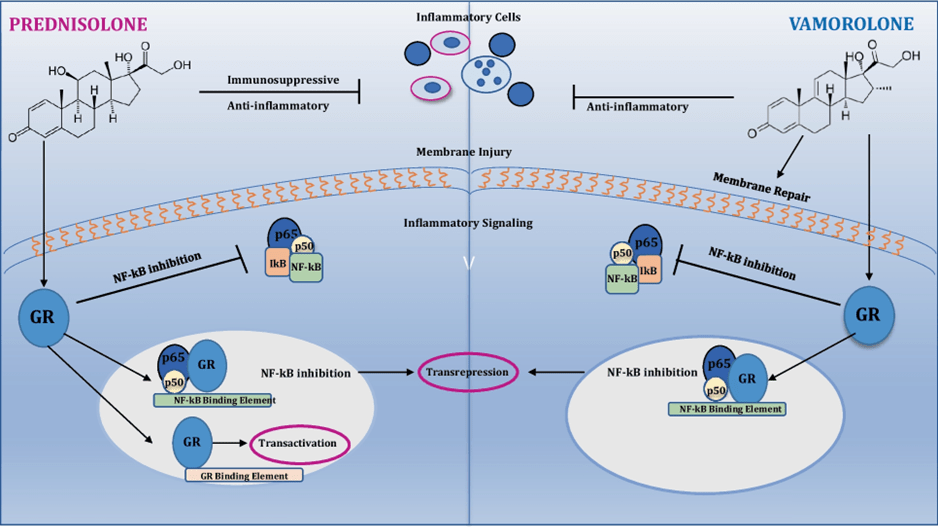
Corticosteroids (CSCs), the current standard of care (SoC) for DMD, are associated with several adverse events (AEs) with long-term use. For instance, prednisone and deflazacort are associated with AEs such as:
- Weight gain
- Stunting of grwoth
- Osteoporosis
- Mood disturbances
- Adrenal insufficiency
Therefore, there remains a clear unmet need for new treatments, which retain the efficacy of CSCs but reduces their AEs, for patients with DMD.
A potential solution: Vamolorone2
Vamolorone is a first-in-class dissociative steroidal anti-inflammatory drug designed to retain the efficacy of CSCs while reducing their associated toxicities.
Outcomes from a pivotal study in DMD2
A randomised, double-blind, multicentre study evaluated the efficacy and safety of vamolorone vs. placebo and prednisone in boys with DMD who were CSC-naïve.

Meeting its secondary endpoints, vamolorone also demonstrated numerical improvements in the 6-minute walk test (6MWT) and the time to run/walk 10 metre test (TTRW) vs. placebo, consistent with the primary endpoint.
In terms of safety, vamolorone reduced CSC-associated AEs, preserving growth and maintaining comparable BMI and overall tolerability vs. prednisone.
Clinical implications of these results
- Vamolorone may provide clinicians an alternative CSC treatment for patients with DMD with anti-inflammatory efficacy
- Flexibility across the 2–6 mg/kg dose range enables individualised dosing based on clinical observations and patient needs
- Comparable TEAEs rates across treatment groups suggest vamolorone may be a well-tolerated treatment option for patients with DMD in clinical practice
- The preservation of linear growth and stable BMI may help patients with DMD reduce growth-related complications associated with prolonged CSC use.
Key takeaway: Vamolorone may have the potential to provide therapeutic benefit with improved tolerability vs. SoC CSCs for patients with DMD.
References
1. Muscular Dystrophy UK. Duchenne Muscular Dystrophy (DMD). Available at: https://www.musculardystrophyuk.org/conditions/a-z/duchenne-muscular-dystrophy-dmd/. Accessed 2025.
2. Guglieri M, et al. JAMA Neurol. 2022;79(10):1005–1014.


Leave a comment